Background:
Disease recurrence is the main cause of transplantation failure for high-risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Exploring innovative approaches to prevent relapse in AML and MDS after Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is in urgent need.
Both bcl-2 inhibitor venetoclax (VEN) and azacitidine (AZA) possess significant antitumor activity effects against myeloid neoplasms. The efficacy and safety of AZA alone as maintenance therapy in the posttransplant setting have been reported but the results are controversial. The combination of VEN and AZA has resulted in high response rates in treating AML patients. We conducted a prospective clinical trial to compare the utility and safety of the combination of VEN and AZA (VEN/AZA) and AZA alone as maintenance treatment in high-risk AML and MDS in the post-transplant setting.
Methods:
This is a prospective, multicenter, randomized clinical trial. Patients with AML or MDS who were evaluated as high risk of relapse and underwent allo-HSCT were randomly assigned into VEN/AZA group and AZA group by gender, age, and disease type based on the central randomization system. AZA was administered by subcutaneous injection for 5 days at a dose of 50 mg/m2. VEN was administrated at the dosage of 200 mg daily from day 1 to day 7. VEN/AZA maintenance therapy regimen comprised AZA and VEN used alternately every month. AZA alone group was administrated AZA every 2 months. Maintenance treatment lasted at least 1 year after transplantation unless relapse occurred. The primary endpoint of the study included overall survival (OS), disease-free survival (DFS), and cumulative incidence of relapse (CIR). The second endpoints included adverse events (AEs), nonrelapse mortality (NRM), incidence of acute graft versus host disease (aGVHD), and chronic GVHD (cGVHD).
Results:
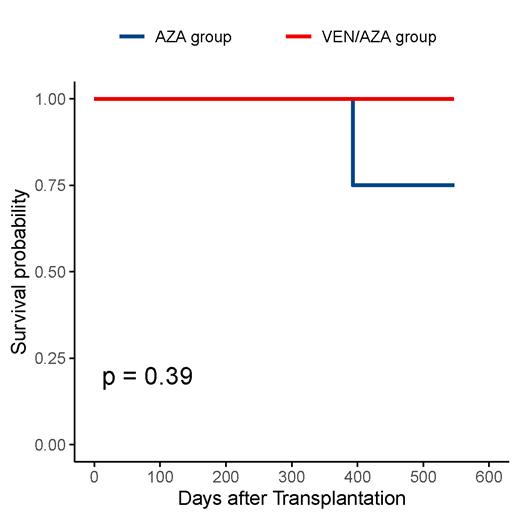
From June 2022 to June 2023, 24 patients were given the study protocol. 9 patients (6 AML, 3 MDS) received VEN/AZA maintenance and 15 (7 AML, 8 MDS) patients received only AZA maintenance. The median initial time to receive maintenance treatment was 3.67 months after transplantation. The median follow-up time was 11.30 (95%CI, 8.18-14.42) months. Only 1 patient relapsed and died 3 months after relapse on the study. The overall survival (OS) and the cumulative incidence of relapse (CIR) at 500 days were 100% vs 75.0% (95% CI, 42.59%-100%, p=0.39) and 0% vs 12.5% (95% CI, 0.48%-44.48%, p=0.39) for VEN/AZA and AZA group, respectively. The most common adverse effects (AEs) were hematological toxicities. Grade 3 or 4 hematological toxicities were observed in 5/9 (55.5%) in the VEN/AZA and 7/15 (46.7%) in the AZA group.
Conclusions:
Our results suggested that both VEN/AZA and AZA maintenance therapy were tolerated and the combination of VEN and AZA as maintenance therapy may be feasible and safe in the posttransplant setting to prevent relapse for high-risk AML and MDS patients.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Venetoclax and azacitidine were used as maintenance treatment in AML and MDS patients after allo-HSCT.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal